CAN YOU IMAGINE A LIFE without water? Of course not, because water is essential to sustain life. Likewise, body fluids are vital to maintain normal body functioning. The body reacts to internal and environmental changes by adjusting vital functions to keep fluids and electrolytes in balance, maintaining homeostasis. This article will explore how fluid acts within the body and discuss when and why various I.V. fluids can be used to maintain homeostasis. Subsequent articles in this series will discuss specific electrolyte imbalances. Unless otherwise specified, information applies to adults, not pediatric patients. Water water everywhere Solutions are comprised of fluid (the solvent) and particles (the solute) dissolved in the fluid. Water is the body's primary fluid and is essential for proper organ system functioning and survival. Although people can live several weeks without food, they can survive only a few days without water. Water has many functions in the body; for example, it | |||
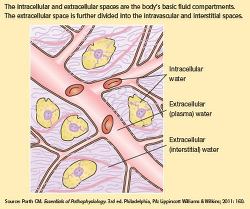
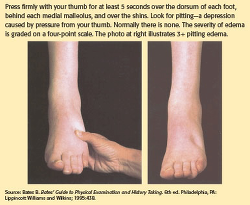
* facilitates the elimination of wastes through the kidneys, gastrointestinal (GI) tract, skin, and lungs. * regulates body temperature through evaporation from the skin. Water is gained and lost from the body every day. For the body to maintain normal function, the intake and output of fluid should remain fairly equal. We obtain water through drinking fluids and the metabolism of nutrients obtained from eating foods. Fluid intake is regulated by the thirst mechanism in the brain. This mechanism is stimulated when blood fluid volume decreases. Increased osmolality stimulates the thirst center, triggering the impulse to increase fluid intake. Water is lost from the body through the kidneys, GI tract, lungs, and skin. Losses from the kidneys and GI tract are known as sensible lossesbecause they can be measured. Insensible losses describe water loss that can't be measured, including losses through the skin from evaporation and through the lungs from respiration. Two main fluid compartments Fluids within the body are contained in two basic compartments, intracellular and extracellular. Cell membranes and capillary walls separate the two fluid compartments. See Two basic fluid compartments. The intracellular fluid compartment, which consists of fluid contained within all of our body cells, is the larger of the two compartments. The extracellular fluid compartment contains all the fluids outside the cells and is further divided into two major subcomponents: intravascular fluidcontained in blood vessels and interstitial fluid found in the tissue spaces. The intracellular, intravascular, and interstitial spaces are the major fluid compartments in the body. A third category of the extracellular fluid compartment is the transcellular compartment, which includes cerebrospinal fluid and fluid contained in body spaces such as the pleural cavity and joint spaces. Because transcellular fluids don't normally contribute significantly to fluid balance, they're beyond the scope of this article. How much of you is water? The amount of water in the body varies depending on age, gender, and body build. In nonobese adults, intracellular fluid constitutes approximately 40% of body weight, and extracellular fluid, 20%. (See How body fluid is distributed.) Lean body muscle mass is rich in water, while adipose tissue has a lower percentage of water content. Because of this, someone who's overweight or obese has a lower percentage of water overall compared with someone who's lean and muscular. Similarly, women typically have a lower percentage of total body water than men due to a higher percentage of body fat. Older adults tend to have a lower concentration of water overall, due to an age-related decrease in muscle mass. Conversely, children tend to have a higher percentage of water weight-as much as 80% in a full-term neonate. Fluids don't remain static within body compartments; instead, they move continuously among them to maintain homeostasis. Cell membranes are semipermeable, meaning they allow fluid and some solutes (particles dissolved in a solution) to pass through. Fluids and electrolytes move between compartments via passive and active transport. Passive transport occurs when no energy is required to cause a shift in fluid and electrolytes. Diffusion, osmosis, and filtration are examples of passive transport mechanisms that cause body fluid and electrolyte movement. Osmolality and osmolarity are two similar terms that are often confused. Osmolality, which is usually used to describe fluids inside the body, refers to the solute concentration in fluid by weight: the number of milliosmols (mOsm) in a kilogram (kg) of solution. Osmolarity refers to the solute concentration in fluid by number of mOsm per liter (L) of solution. Because 1 L of water weighs 1 kg, the normal ranges are the same and the terms are often used interchangeably. Changes in the level of solute concentration influence the movement of water between the fluid compartments. The normal osmolality for plasma and other body fluids varies from 270 to 300 mOsm/L. Optimal body function occurs when the osmolality of fluids in all the body compartments is close to 300 mOsm/L. When body fluids are fairly equivalent in this particle concentration, they're said to be isotonic. Fluids with osmolalities less than 270 mOsm/L are hypotonic in comparison with isotonic fluids, and fluids with osmolalities greater than 300 mOsm/L are hypertonic. Tonicity of I.V. fluids will be discussed in detail later in this article. Through the use of mechanisms such as thirst, the renin-angiotensin-aldosterone system, antidiuretic hormone, and atrial natriuretic peptide, the body works to maintain appropriate fluid and electrolyte levels and to prevent imbalances within the body. When an imbalance occurs, you must be able to identify the cause of the problem and monitor the patient during treatment. Crystalloids vs. colloids One of the methods for treating fluid and electrolyte alterations is the infusion of I.V. solutions, which have distinctive differences in composition that affect how the body reacts to and utilizes them. When administering I.V. therapy, you need to understand the nature of the solution being initiated and how it will affect your patient's condition. I.V. solutions for fluid replacement may be placed in two general categories: colloids and crystalloids. Colloids contain large molecules that don't pass through semipermeable membranes. When infused, they remain in the intravascular compartment and expand intravascular volume by drawing fluid from extravascular spaces via their higher oncotic pressure. We'll discuss colloids in detail later. Crystalloids are solutes capable of crystallization that are easily mixed and dissolved in a solution. The solutes may be electrolytes or nonelectrolytes, such as dextrose. Crystalloid solutions contain small molecules that flow easily across semipermeable membranes, allowing for transfer from the bloodstream into the cells and body tissues. This may increase fluid volume in both the interstitial and intravascular spaces. Crystalloid solutions are distinguished by their relative tonicity (before infusion) in relation to plasma. Tonicity refers to the concentration of dissolved molecules held within the solution. The following sections discuss isotonic, hypotonic, and hypertonic crystalloid solutions in detail. ISOTONIC FLUIDS A solution is isotonic when the concentration of dissolved particles is similar to that of plasma. Isotonic solutions have an osmolality of 250 to 375 mOsm/L. With osmotic pressure constant both inside and outside the cells, the fluid in each compartment remains within its compartment (no shift occurs) and cells neither shrink nor swell. Because isotonic solutions have the same concentration of solutes as plasma, infused isotonic solution doesn't move into cells. Rather, it remains within the extracellular fluid compartment and is distributed between the intravascular and interstitial spaces, thus increasing intravascular volume. Types of isotonic solutions include 0.9% sodium chloride (0.9% NaCl), lactated Ringer's solution, 5% dextrose in water (D5W), and Ringer's solution. A solution of 0.9% sodium chloride is simply salt water, and contains only water, sodium (154 mEq/L), and chloride (154 mEq/L). It's often called "normal saline solution" because the percentage of sodium chloride dissolved in the solution is similar to the usual concentration of sodium and chloride in the intravascular space. Because water goes where sodium goes, 0.9% sodium chloride increases fluid volume in extracellular spaces. It's administered to treat low extracellular fluid, as in fluid volume deficit from hemorrhage, severe vomiting or diarrhea, and heavy drainage from GI suction, fistulas, or wounds. Conditions commonly treated with 0.9% sodium chloride include shock, mild hyponatremia, metabolic acidosis (such as diabetic ketoacidosis), and hypercalcemia; patients requiring a fluid challenge may also benefit from 0.9% sodium chloride solution. It's the fluid of choice for resuscitation efforts. In addition, it's the only fluid used with administration of blood products. Remember that because 0.9% sodium chloride replaces extracellular fluid, it should be used cautiously in certain patients, such as those with cardiac or renal disease, because of the potential for fluid volume overload. Lactated Ringer's (LR), also known as Ringer's lactate or Hartmann solution, is the most physiologically adaptable fluid because its electrolyte content is most closely related to the composition of the body's blood serum and plasma. Because of this, LR is another choice for first-line fluid resuscitation for certain patients, such as those with burn injuries. It contains 130 mEq/L of sodium, 4 mEq/L of potassium, 3 mEq/L of calcium, and 109 mEq/L of chloride. LR doesn't provide calories or magnesium, and has limited potassium replacement. LR is used to replace GI tract fluid losses, fistula drainage, and fluid losses due to burns and trauma. It's also given to patients experiencing acute blood loss or hypovolemia due to third-space fluid shifts. Both 0.9% sodium chloride and LR may be used in many clinical situations, but patients requiring electrolyte replacement (such as surgical or burn patients) will benefit more from an infusion of LR. LR is metabolized in the liver, which converts the lactate to bicarbonate. As an alkalinizing solution, LR is often administered to patients who have metabolic acidosis. Don't give LR to patients who can't metabolize lactate for some reason, such as those with liver disease or those experiencing lactic acidosis. Because a normal liver will convert it to bicarbonate, LR shouldn't be given to a patient whose pH is greater than 7.5. Because it does contain some potassium, use caution in patients with renal failure. Ringer's solution, like LR, contains sodium, potassium, calcium, and chloride in similar concentrations (147 mEq/L of sodium, 4 mEq/L of potassium, 4 mEq/L of calcium, and 156 mEq/L of chloride). But it doesn't contain lactate. Ringer's solution is used in a similar fashion as LR, but doesn't have the contraindications related to lactate. However, because it's not an alkalizing agent, it may not be indicated for patients with metabolic acidosis. D5W is unique in that it may be categorized as both an isotonic and a hypotonic solution. The amount of dextrose in this solution makes its initial tonicity similar to that of intravascular fluid, making it an isotonic solution. But dextrose (in this concentration) is rapidly metabolized by the body, leaving no osmotically active particles in the plasma. D5W provides free water: free, unbound water molecules small enough to pass through membrane pores to the intracellular and extracellular spaces. This smaller size allows the molecules to pass more freely between compartments, thus expanding both compartments simultaneously.The free water initially dilutes the osmolality of the extracellular fluid; once the cell has used the dextrose, the remaining saline and electrolytes are dispersed as an isotonic electrolyte solution, providing additional hydration for the extracellular fluid compartment. Dextrose solutions also provide free water for the kidneys, aiding renal excretion of solutes. Because it provides free water following metabolism, D5W is also considered a hypotonic solution. D5W is basically a sugar water solution that provides 170 calories per liter, but it doesn't replace electrolytes. However, it's appropriate to treat hypernatremia because it dilutes the extra sodium in extracellular fluid. D5W shouldn't be used in isolation to treat fluid volume deficit because it dilutes plasma electrolyte concentrations. It's also contraindicated in these clinical circumstances: * for resuscitation, because the solution won't remain in the intravascular space. * in the early postoperative period, because the body's reaction to the surgical stress may cause an increase in antidiuretic hormone secretion. * in patients with known or suspected increased intracranial pressure (ICP) due to its hypotonic properties following metabolism. Although it supplies some calories, D5W doesn't provide enough nutrition for prolonged use. Nursing considerations for isotonic solutions Be aware that patients being treated for hypovolemia can quickly develop hypervolemia (fluid volume overload) following rapid or overinfusion of isotonic fluids. Document baseline vital signs, edema status, lung sounds, and heart sounds before beginning the infusion, and continue monitoring during and after the infusion. Frequently assess the patient's response to I.V. therapy, monitoring for signs and symptoms of hypervolemia, such as hypertension, bounding pulse, pulmonary crackles, dyspnea/shortness of breath, peripheral edema, jugular venous distention (JVD), and extra heart sounds, such as S3. Monitor intake and output, hematocrit, and hemoglobin. Elevate the head of bed at 35 to 45 degrees, unless contraindicated. If edema is present, elevate the patient's legs. Note if the edema is pitting or nonpitting and grade pitting edema. For an example, see Checking for pitting edema. Also monitor for signs and symptoms of continued hypovolemia, including urine output of less than 0.5 mL/kg/hour, poor skin turgor, tachycardia, weak, thready pulse, and hypotension. Educate patients and their families about signs and symptoms of volume overload and dehydration, and instruct patients to notify their nurse if they have trouble breathing or notice any swelling. Instruct patients and families to keep the head of the bed elevated (unless contraindicated). HYPOTONIC FLUIDS Compared with intracellular fluid (as well as compared with isotonic solutions), hypotonic solutions have a lower concentration, or tonicity, of solutes (electrolytes). Hypotonic I.V. solutions have an osmolality less than 250 mOsm/L. Infusing a hypotonic solution into the vascular system causes an unequal solute concentration among the fluid compartments. The infusion of hypotonic crystalloid solutions lowers the serum osmolality within the vascular space, causing fluid to shift from the intravascular space to both the intracellular and interstitial spaces. These solutions will hydrate cells, although their use may deplete fluid within the circulatory system. Types of hypotonic fluids include 0.45% sodium chloride (0.45% NaCl), 0.33% sodium chloride, 0.2% sodium chloride, and 2.5% dextrose in water. Hypotonic solutions assist with maintaining daily body fluid requirements, but don't contain any electrolytes (except for sodium and chloride) or calories (except for D5W, which is also considered a hypotonic solution after metabolism). Administering hypotonic saline solutions also helps the kidneys excrete excess fluids and electrolytes. All these solutions provide free water, sodium, and chloride, and replace natural fluid losses. In addition, the solution containing dextrose offers a low level of caloric intake. Nursing considerations for hypotonic solutions Hypotonic fluids are used to treat patients with conditions causing intracellular dehydration, such as diabetic ketoacidosis, and hyperosmolar hyperglycemic state, when fluid needs to be shifted into the cell. Be aware of how the fluid shift will affect various body systems. The lower concentration of solute within the vascular bed will shift the fluid into the cells and also into the interstitial spaces. Use caution when infusing hypotonic solutions; the decrease in vascular bed volume can worsen existing hypovolemia and hypotension and cause cardiovascular collapse. Monitor patients for signs and symptoms of fluid volume deficit as fluid is "pulled back" into the cells and out of the vascular bed. In older adult patients, confusion may also be an indicator of a fluid volume deficit. Instruct patients to inform a nurse if they feel dizzy or just "don't feel right." Never give hypotonic solutions to patients who are at risk for increased ICP because of a potential fluid shift to the brain tissue, which can cause or exacerbate cerebral edema. In addition, don't use hypotonic solutions in patients with liver disease, trauma, or burns due to the potential for depletion of intravascular fluid volume. HYPERTONIC SOLUTIONS Compared with intracellular fluid (as well as with isotonic solutions), hypertonic solutions have a higher tonicity or solute concentration, causing an unequal pressure gradient between the inside and outside of the cells. Hypertonic fluids have an osmolarity of 375 mOsm/L or higher. The osmotic pressure gradient draws water out of the intracellular space, increasing extracellular fluid volume. Because of this property, hypertonic solutions are used as volume expanders. Hypertonic solutions may be prescribed for patients with severe hyponatremia. Patients with cerebral edema may also benefit from an infusion of hypertonic sodium chloride. Hypertonic sodium chloride solutions contain a higher concentration of sodium and chloride than that normally contained in plasma. Examples include 3% sodium chloride (3% NaCl), with 513 mEq/L of sodium and chloride, and 5% sodium chloride (5% NaCl), with 855 mEq/L of sodium and chloride. As the infusion of these hypertonic solutions raise the sodium level in the bloodstream, osmosis comes into play, removing fluid from the intracellular space, and shifting it into the intravascular and interstitial spaces. These solutions are highly hypertonic and should be used only in critical situations to treat hyponatremia. Give them slowly and cautiously to avoid intravascular fluid volume overload and pulmonary edema. When dextrose is added to isotonic or hypotonic solutions, the net result can be a slightly hypertonic solution due to the higher solute concentration. Thus, adding D5W to sodium chloride solutions (such as 5% dextrose and 0.45% sodium chloride, and 5% dextrose and 0.9% sodium chloride) or to lactated Ringer's solutions such as D5LR will provide the same electrolytes already discussed for each of those solutions, with the addition of calories. Plain glucose solutions with a concentration higher than 5%, such as 10% dextrose in water (D10W), are also considered hypertonic. D10W provides free water and calories (340 per liter), but not electrolytes. Twenty percent dextrose in water (D20W) is an osmotic diuretic, meaning the fluid shift it causes between various compartments promotes diuresis. Fifty percent dextrose in water (D50W) is a highly concentrated sugar solution. It's administered rapidly via I.V. bolus to treat patients with severe hypoglycemia. Nursing considerations for hypertonic solutions Maintain vigilance when administering hypertonic saline solutions because of their potential for causing intravascular fluid volume overload and pulmonary edema. Hypertonic sodium chloride solutions should be administered only in high acuity areas with constant nursing surveillance for potential complications. Hypertonic sodium chloride shouldn't be given for an indefinite period of time. Prescriptions for their use should state the specific hypertonic fluid to be infused, the total volume to be infused and infusion rate, or the length of time to continue the infusion. As an additional precaution, many institutions store hypertonic sodium chloride solutions apart from regular floor stock I.V. fluids, so they must be ordered separately from the pharmacy. Monitor serum electrolytes and assess for signs and symptoms of hypervolemia. Because hypertonic solutions can cause irritation, damage, and thrombosis of the blood vessel, some of these solutions shouldn't be administered peripherally. The Infusion Nurses Society states that "[p]arenteral nutrition solutions containing final concentrations exceeding 10% dextrose should be administered through a central vascular access device with the tip located in the central vasculature, preferably the subclavian/right atrium junction for adults." Instruct patients to notify a nurse if they develop breathing difficulties or if they feel their heart is beating very fast. Hypertonic solutions shouldn't be given to patients with cardiac or renal conditions who are dehydrated. These solutions affect renal filtration mechanisms and can cause hypervolemia. Patients with conditions causing cellular dehydration, such as diabetic ketoacidosis shouldn't be given hypertonic solutions, because it will exacerbate the condition. Why colloid solutions stay put Unlike crystalloids, colloids contain molecules too large to pass through semipermeable membranes, such as capillary walls. Because they remain in the intravascular compartment, they're also known as volume expanders or plasma expanders. Examples include albumin, dextrans, and hydroxyethylstarches. Colloids expand intravascular volume by drawing fluid from the interstitial spaces into the intravascular compartment through their higher oncotic pressure. They have the same effect as hypertonic crystalloids of increasing intravascular volume, but require administration of less total volume compared with crystalloids. In addition, colloids have a longer duration of action than crystalloids because the molecules remain within the intravascular space longer. The effects of colloids can last for several days if capillary wall linings are intact and working properly. Colloids are indicated for patients exhibiting hypoproteinemia, and malnourished states, as well as for those who require plasma volume expansion but who can't tolerate large infusions of fluid. Patients undergoing orthopedic surgery or reconstructive procedures with an elevated potential for thrombus formation may also benefit from colloid solutions. Five percent albumin (Human albumin solution) is one of the most commonly utilized colloid solutions. It contains plasma protein fractions obtained from human plasma and works to rapidly expand the plasma volume. It's used for volume expansion, moderate protein replacement, and achievement of hemodynamic stability in shock states. Albumin is also available in a 25% solution, which is much more hypertonic and can draw about four times its volume from the interstitial fluid into the vascular compartment within 15 minutes of administration. Albumin is considered a blood transfusion product and requires all the same nursing precautions used when administering other blood products. It can be expensive and its availability is limited to the supply of human donors. Albumin is, however, contraindicated in patients with the following conditions: severe anemia, heart failure, or a known sensitivity to albumin. In addition, angiotensin-converting enzyme inhibitors should be withheld for at least 24 hours before administering albumin because of the risk of atypical reactions, such as flushing and hypotension. A study was conducted during 2001-2003 called the Saline versus Albumin Fluid Evaluation (SAFE) study. This study compared the use of albumin and saline for ICU patients requiring fluid resuscitation. Among 6997 patients studied, 3497 received 4% albumin solution and 3500 received 0.9% sodium chloride solution. The aim of the study was to determine if one fluid was better than the other for preventing death. After 28 days, researchers found similar outcomes in both groups. Because neither solution has proven clearly superior, healthcare providers use their judgment to decide which fluid to administer to critically ill patients in the ICU. Besides albumin, several synthetic colloid preparations are available for patient use. Low-molecular weight dextran (LMWD) and high-molecularweight dextran(HMWD) are synthetic plasma expanders infused to draw water into the intravascular space. * LMWD contains polysaccharide molecules that behave like colloids with an average molecular weight of 40,000 (dextran 40). It contains no electrolytes and is used for volume expansion and support. LMWD is used for early fluid replacement and to treat shock related to vascular volume loss, such as that produced by burns, hemorrhage, surgery, or trauma. It's used to prevent venous thromboembolism during surgical procedures, because its mechanism of action is to prevent the sludging of blood. LMWD is contraindicated in patients with thrombocytopenia, hypofibrinogenemia, and hypersensitivity to dextran. * HMWD contains polysaccharide molecules with an average molecular weight of 70,000 (available as dextran 70) or 75,000 (available as dextran 75). It also contains no electrolytes. HMWD shouldn't be given to patients in hemorrhagic shock. Dextran solutions are available in either saline or glucose solutions. Dextran interferes with lab blood crossmatching, so if a type and cross is anticipated, draw the patient's blood before administering dextran. Dextran may interfere with some other blood tests and may also cause anaphylactoid reactions. Hydroxyethalstarches, such as hetastarch (6%) and hespan, are another form of hypertonic synthetic colloids used for volume expansion. They contain 154 mEq/L of sodium and chloride and are used for hemodynamic volume replacement following major surgery and to treat major burns. Synthetic colloid preparations are less expensive than albumin and their effects can last 24 to 36 hours. Unlike other colloids, hetastarch doesn't interfere with blood typing or crossmatching. Hetastarch is contraindicated in patients with liver disease and severe cardiac and renal disorders. It may also cause a severe anaphylactoid reaction. Nursing considerations for colloids Because colloids pull fluids from the interstitial space to the vascular space, the patient is at risk for developing fluid volume overload. If the patient's fluid imbalance doesn't respond to either crystalloids or colloids, blood transfusions or other treatment may be necessary. As for blood products, use an 18-gauge or larger needle to infuse colloids. Monitor the patient for signs and symptoms of hypervolemia, including increased BP, dyspnea, crackles in the lungs, JVD, edema, and bounding pulse. Closely monitor intake and output. Colloid solutions can interfere with platelet function and increase bleeding times, so monitor the patient's coagulation indexes. Elevate the head of bed unless contraindicated. Anaphylactoid reactions are a rare but potentially lethal adverse reaction to colloids. Take a careful allergy history from patients receiving colloids (or any other drug or fluid), asking specifically if they've ever had a reaction to an I.V. infusion. Use best practices for optimal outcomes No matter what I.V. fluid you're administering, follow best practices to ensure optimal response to therapy and prevent complications. For example, assess and document baseline vital signs, heart and lung sounds, and fluid volume status. As with any drug, make sure you're familiar with the type of fluid being administered, the rate and duration of the infusion, the fluid's effects on the body, and potential adverse reactions. Throughout therapy, monitor the patient's response to treatment, watching closely for any signs and symptoms of hypervolemia or hypovolemia. Monitor lab values to assess kidney function and fluid status. Regularly check the venous access site for signs of infiltration, inflammation, infection, or thrombosis. Educate the patient and the family about the prescribed therapy, including potential complications and symptoms that require immediate attention. Crucial balancing act Maintaining fluid and electrolyte balance is essential for life. Future articles in this series will discuss how to assess for specific imbalances and intervene appropriately. |


No comments:
Post a Comment